Oligonucleotides are short strands of DNA or RNA that scientists use to influence biological processes at the genetic level. They have become central to modern medicine, playing key roles in antisense therapies, RNA interference drugs, and even vaccines.
For example, the New England Journal of Medicine has highlighted how antisense oligonucleotides can alter gene expression in rare neurological diseases.
Because these molecules hold so much therapeutic promise, how they are made matters greatly.
A recently published patent Novel Processes For The Production Of Oligonucleotides (WO2018011067A2), describes new strategies for improving oligonucleotide synthesis, offering solutions to long-standing challenges of purity, cost, and scalability.
These innovations aim to make the production of oligonucleotides faster, cleaner, and more reliable, something both researchers and patients stand to benefit from.
The current standard for making oligonucleotides is called solid-phase synthesis, first developed in the 1980s. This method, which the biotechnology company Bachem describes as a “stepwise assembly line,” builds DNA or RNA strands one nucleotide at a time. Each cycle involves four main steps:
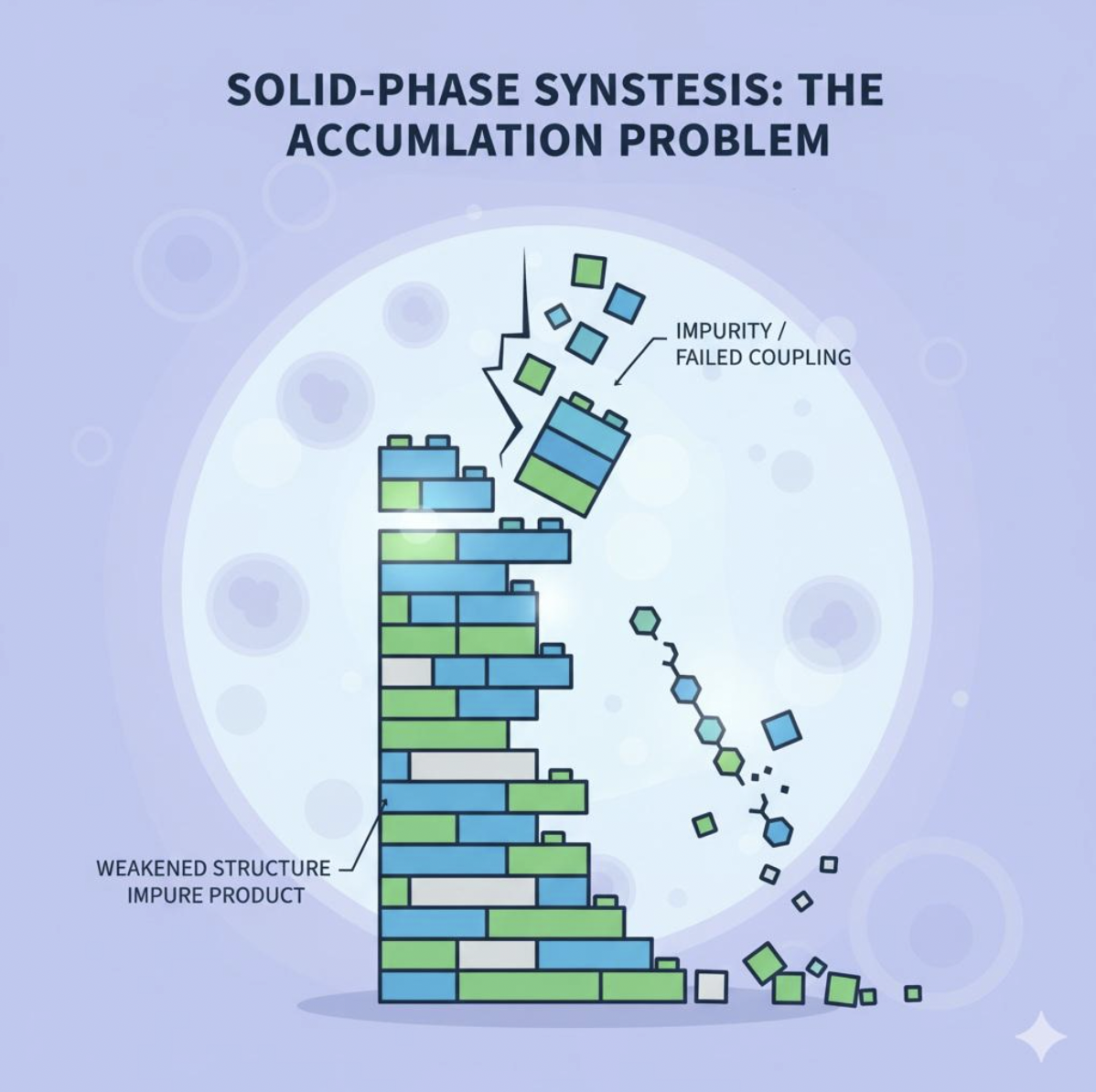
While this method has enabled decades of drug discovery, it is not without flaws. According to a 2023 review in Journal of Pharmaceutical and Biomedical Analysis, even small inefficiencies in a single step can accumulate into significant impurities in the final product. These errors lower quality and can compromise safety.
Another concern is waste. Solid-phase synthesis consumes large amounts of acetonitrile and other solvents, which the American Chemical Society has flagged as a major sustainability challenge for the oligonucleotide industry. And because the process was designed for lab-scale use, scaling up to commercial production often requires costly adjustments and custom engineering.
A useful analogy is building a tower out of Lego bricks: if just one block is misaligned, the entire tower becomes unstable. In the same way, one failed coupling step can weaken the integrity of an oligonucleotide chain.
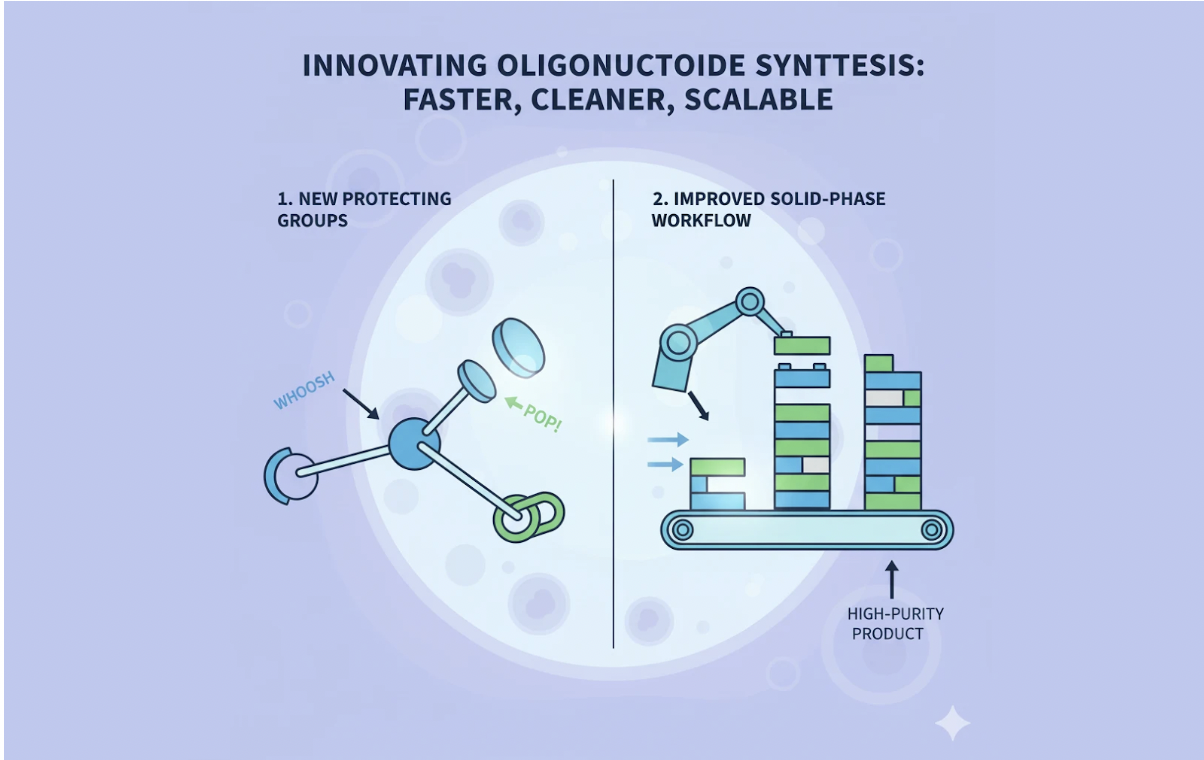
The patent addresses these problems through two key improvements:
During synthesis, protecting groups act as temporary “caps” that prevent unwanted chemical reactions. Traditional protecting groups sometimes fail to come off cleanly, leaving behind fragments that lower purity. The new protecting groups described in WO2018011067A2 are designed to detach more efficiently, reducing side products. A related study published in Science Advances in 2021 emphasized how cleaner deprotection chemistry can significantly increase the success rate of oligonucleotide assembly, lending weight to the approach in this patent.
The patent also suggests changes in how each cycle of synthesis is managed, aiming to improve coupling efficiency and reduce wasted reagents. By making the step-by-step process more reliable, the method can yield a higher percentage of the desired product. Researchers writing in Nucleic Acids Research have shown that even small improvements in coupling efficiency compound across dozens of synthesis cycles, resulting in much cleaner final products.
In plain terms: these innovations act like better tools for both capping and joining the building blocks, leading to a smoother production line. The outcome is a faster, cleaner, and more scalable synthesis process, critical if oligonucleotides are to be produced at the level required for widespread therapeutic use.
For scientists, more efficient synthesis means time saved and fewer failed experiments. When impurities are reduced, researchers can work with cleaner samples, which makes results more reliable. A 2020 article in Nature Reviews Drug Discovery noted that one of the biggest bottlenecks in oligonucleotide research is inconsistent product quality, something innovations like new protecting groups directly address.
For pharmaceutical companies, these improvements make it easier to scale production to clinical and commercial levels. The U.S. Food and Drug Administration (FDA) requires strict purity standards for any therapeutic product, and cleaner synthesis methods can shorten the path to regulatory approval. Service providers offering specialized oligonucleotide synthesis services play a key role here, helping drug developers meet regulatory requirements while keeping timelines efficient.
For patients, the impact is ultimately felt in faster access to new treatments. Oligonucleotides are already being tested for cancer, viral infections, and rare genetic conditions. With better manufacturing, these therapies could move more quickly from research labs into clinical trials and, eventually, into pharmacies and hospitals.
The broader biotech industry is moving in two directions: refining chemical synthesis while also exploring enzymatic synthesis. Enzymatic approaches, using biological catalysts rather than chemicals, are gaining attention for being more environmentally friendly, as highlighted in a 2022 Nature Biotechnology article. Still, chemical synthesis remains the gold standard today, and patents like WO2018011067A2 show that researchers are still finding ways to make it more efficient and scalable.
Another important trend is the growth of contract development and manufacturing organizations (CDMOs) specializing in nucleic acids. According to a market analysis published in Drug Discovery Today, demand for oligonucleotide manufacturing is outpacing in-house capabilities at many pharma companies, leading to greater reliance on external partners. Innovations in synthesis chemistry, such as those described in the patent, make CDMOs more competitive and capable of meeting rising demand.
Sustainability is also becoming a key focus. The American Chemical Society has published multiple calls for greener oligonucleotide production, pointing to the heavy solvent use in current methods. Cleaner reactions and more efficient workflows can help reduce the environmental footprint of this rapidly growing field.
Future production methods will likely combine the precision of chemical synthesis with the eco-friendliness of enzymatic approaches. Researchers writing in Accounts of Chemical Research suggested that hybrid strategies could offer the best of both worlds, efficiency at scale and reduced chemical waste.
Personalized medicine is another frontier. Oligonucleotides can be designed for individual patients, but this only works if synthesis is fast and reliable. With the improvements discussed in this article, the path toward on-demand, patient-specific therapies becomes more realistic. As these therapies progress from laboratory concepts to clinical applications, safety testing through GLP toxicology studies becomes a crucial bridge, ensuring that promising oligonucleotides are not only effective but also safe for human use.
Oligonucleotides are shaping the future of medicine, but their promise depends on how well we can produce them. The innovations outlined in patent WO2018011067A2, new protecting groups and improved solid-phase synthesis, tackle core challenges of yield, purity, and scalability. Backed by supporting research from Nature, Science Advances, and Nucleic Acids Research, these methods point toward a future where oligonucleotide drugs are cheaper, cleaner, and more accessible.
As the industry continues to balance chemical and enzymatic approaches, these incremental improvements could play a crucial role in delivering life-saving therapies to patients faster and more sustainably.
ScienceDirect (2023) – Hybrid support approaches in solid-phase synthesis, European Journal of Medicinal Chemistry. Discusses resin selection impact on synthesis performance.