You ran a CRISPR experiment.
Everything seemed successful—until your signal faded and results grew inconsistent.
Often, the problem isn’t your edit, but the cell line’s instability.
A successful CRISPR knockin isn’t just about inserting DNA. It’s about ensuring that edit stays functional and reliable over time.

For many researchers using CRISPR gene editing, success is often declared after initial confirmation: a positive PCR result, a fluorescent tag, or a functional assay. But short-term validation doesn’t guarantee long-term reliability.
Over time, edited sequences can become silenced, diluted, or even lost as cells divide. Without genomic stability, what starts as a clean result may end in inconsistency, wasting both time and resources.
This is especially critical in experiments that depend on consistent outputs—like protein expression profiling, drug response modeling, or longitudinal studies. In these cases, unstable cell lines mean irreproducible data—and missed discoveries. Imagine investing months in developing a new compound, only to realize your model was expressing the wrong variant all along.
To avoid these pitfalls, scientists must think beyond “successful transfection” and plan for durability from the start.
Instability in CRISPR-edited cell lines is rarely caused by a single factor. More often, it’s the result of a chain reaction—beginning with the editing process itself.
Technically, knock-in projects can suffer from random integration events, incomplete homologous recombination, or residual plasmid expression. If unverified clones slip through screening, these errors become embedded in the workflow.
Biologically, cells react to genome editing with stress responses that can affect viability, gene expression, or cell cycle behavior. Over multiple passages, even a seemingly successful clone can drift in unexpected ways. Epigenetic changes, such as DNA methylation or histone modifications, may also contribute to the silencing of inserted sequences.
Additionally, some genomic loci are inherently unstable. Inserting into repetitive or transcriptionally silenced regions increases the likelihood of methylation, silencing, or recombination. These risks compound when researchers prioritize speed over precision.
Recognizing and addressing these hidden sources early can prevent costly rework and ensure your data is based on models you can trust.
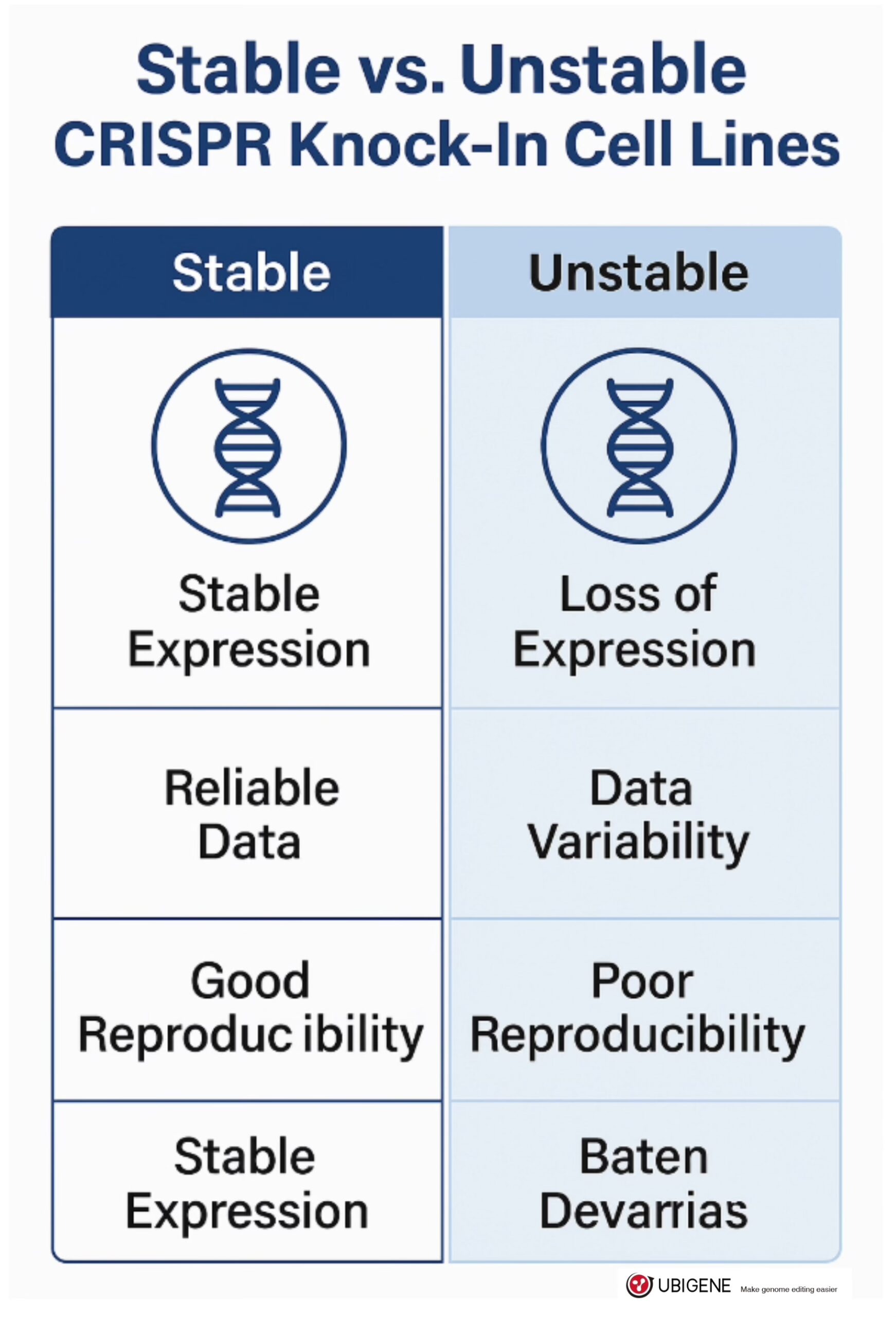
Not all edited cell lines are created equal. A truly stable knock-in model meets three core criteria: genomic precision, expression consistency, and durability over time.
First, precise integration matters. Knock-in events should occur exactly where intended, with no random insertions or partial sequences. This requires both well-designed donor templates and proper validation—typically via junction PCR, Sanger sequencing, and off-target screening.
Second, consistent expression must be maintained across passages. A strong GFP signal on day two is meaningless if it fades by passage six. Stability means the inserted gene behaves as expected under physiological conditions—without drifting, silencing, or rearranging. This can be verified through luciferase assays, Western blots, or single-cell fluorescence tracking.
Third, stability must be heritable. In pooled populations or clonal lines, edits should persist across generations with minimal variance. If you’re preparing cells for functional assays, drug testing, or long-term imaging, this is non-negotiable.
Stable knock-in lines aren’t just scientifically important—they’re operationally efficient. They reduce do-overs, increase confidence, and help ensure your results are worth publishing.
The proprietary EZ-HRex™ platform was designed to enhance homologous recombination and minimize unintended outcomes. It improves knock-in precision through synchronized cell cycle control, optimized donor design, and inhibition of competing DNA repair pathways like NHEJ. The result? Knock-in efficiencies of up to 84% in commonly used lines like HEK293T—and verified success in harder-to-edit cell types.
But technology alone isn’t enough. Ubigene pairs EZ-HRex™ with comprehensive quality control to ensure long-term reliability. This includes:
Additionally, each project receives consulting support from Ubigene’s scientific team, helping researchers design knock-in strategies that prioritize stability from day one. This collaborative approach ensures edits are not only precise—but also dependable across replicates, scalable for screening, and durable for downstream applications such as drug discovery or therapeutic development.
With this full-stack methodology, Ubigene empowers scientists to move beyond trial-and-error toward consistent CRISPR knock-in success.
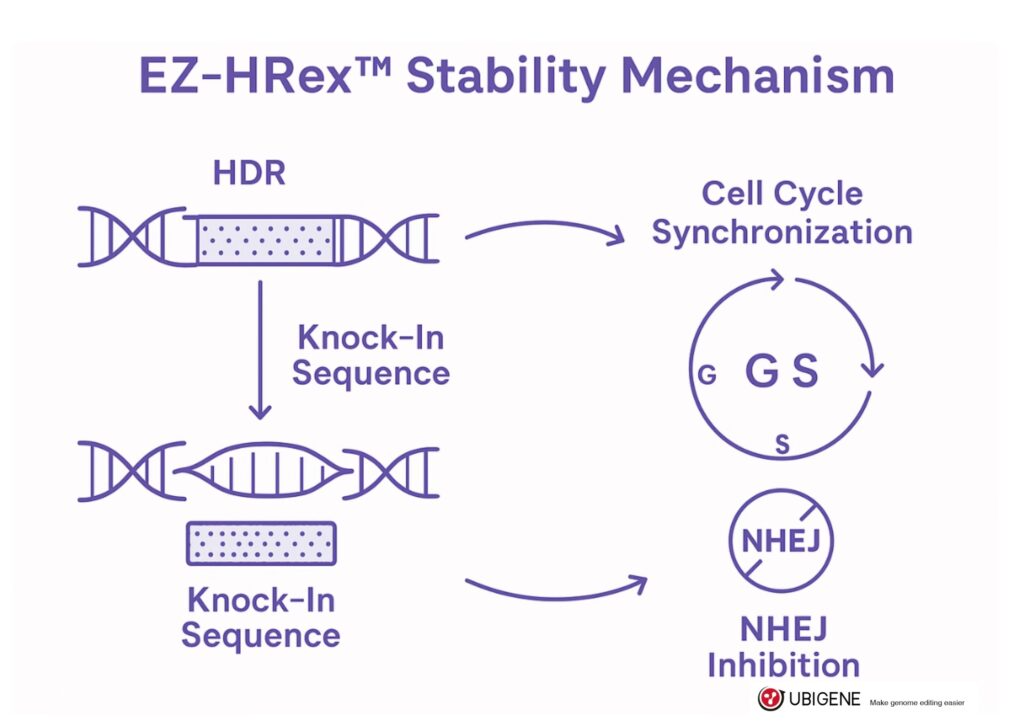
This combination allows researchers to move beyond quick fixes and instead build models that last—models that behave consistently across experiments and support high-impact results.
Whether you’re conducting drug screening, gene function studies, or custom model development, Ubigene’s approach ensures your edited cells are just as reliable six weeks from now as they are today.
In CRISPR research, it’s tempting to celebrate early success—especially when the initial verification checks out. But gene editing isn’t just about getting the right sequence into the genome. It’s about making sure that sequence stays active, stable, and reproducible over time.
That’s where stability becomes the dividing line between quick wins and lasting impact. For projects involving drug screening, disease modeling, or multi-stage assays, only a durable cell line can deliver data you can trust.
So before you start your next knock-in experiment, ask yourself: Are you building a model for today—or one that will still perform tomorrow?
Because in modern functional genomics, it’s not enough to be fast—you need to be consistent. And the true value of a knock-in edit is measured not just in the week it’s made, but in the months it continues to work.
📌 Explore what’s possible with Ubigene’s customized knock-in solutions, powered by EZ-HRex™ and backed by end-to-end validation.
🧬 Whether you need a reporter line, disease model, or complex insertion, Ubigene helps you go beyond editing—toward stability that lasts.